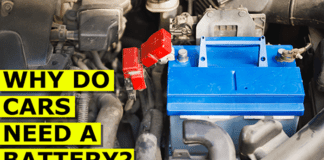
The 12V lead acid car battery. These large and fairly heavy batteries are used in every combustion engine vehicle on the planet. They are an essential part of the vehicle. So what does it do and how does it work? That’s what we will be covering in this article which is sponsored by Squarespace. Head to squarespace.com to start your free trial or use code engineeringmindset to save 10% on websites and domains.
Scroll to the bottom to watch the YouTube tutorial.
What is a Car Battery?
The 12V car battery looks something like this.
This is a lead acid battery. We call it a lead acid battery because inside the unit are lead plates which are submerged into an acid. This creates a chemical reaction which releases energy and provides us with a voltage and current.
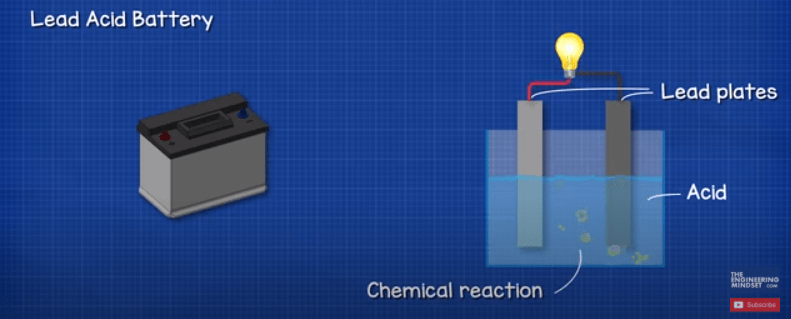
The battery is therefore storing energy in the form of Chemical energy. It doesn’t store electricity. This chemical energy is converted into electrical energy whenever we need it. This battery is also rechargeable, if we supply the battery with electricity then we can reverse the chemical reaction and recharge the battery.
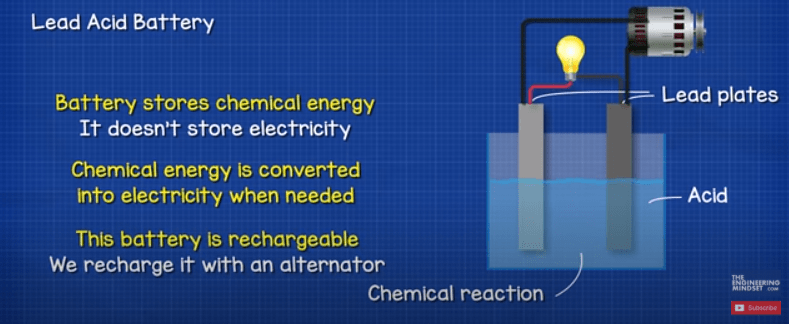
These types of battery can provide large amounts of current, especially compared to the typical, smaller, household alkaline batteries.
We have covered how alkaline batteries work in our previous article, do check that out HERE.
Why is a Battery Used in a Car?
The typical car battery is found in the engine bay of the car. The battery is first used to start the engine and it does this by providing electricity to a small electrical motor known as the starter motor. It also provides electricity to the ignition system to start the combustion of fuel.
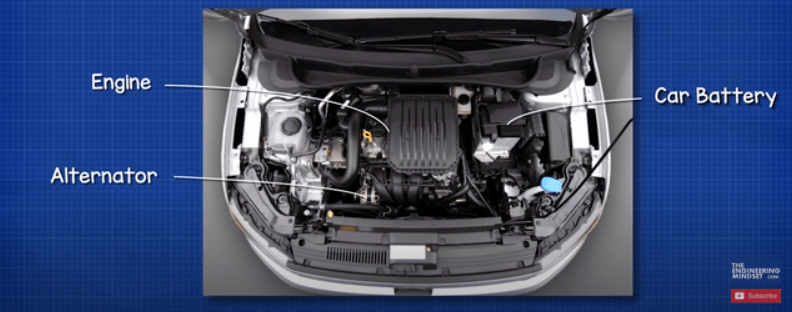
The starter motor engages a small gear onto the flywheel of the motor. It turns this to turn the crankshaft which starts the combustion engine, the small gear then disengages and the engine runs by itself. The starter motor needs to provide a huge amount of force to be able to turn the flywheel, so the starter motor will draw an extremely large current, possibly hundreds of amps, but for only a few seconds. This large current is going to reduce the energy stored in the battery. So we need to top it back up.
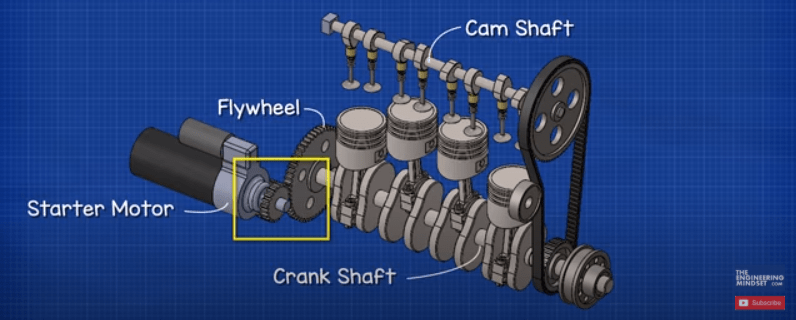
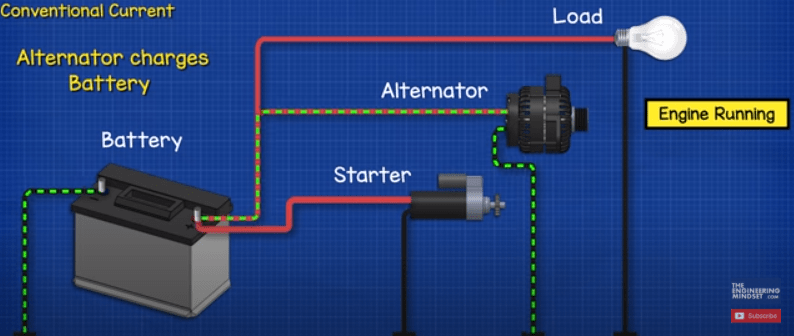
Connected to the engine is an alternator. The alternator is rotated by the engine and as it rotates it generates electricity. This is fed back into the battery to recharge it.

While the engine is running, the alternator recharges the battery, but it also provides the electrical power for things such as the lighting and music system. When the demand for electricity exceeds what the alternator can provide, the battery will provide the additional power, which again drains the battery.
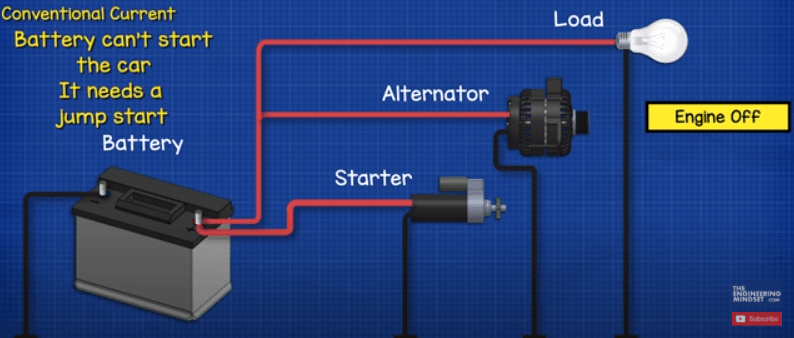
If the engine is switched off, the alternator stops rotating and recharging the battery, so the battery will provide the full electrical power until it’s dead. At this point the battery can’t provide enough electricity to start the engine, so we need to jump start the car.
Main Parts
Let’s have a look at the main parts of a car battery and then we will understand how it works.
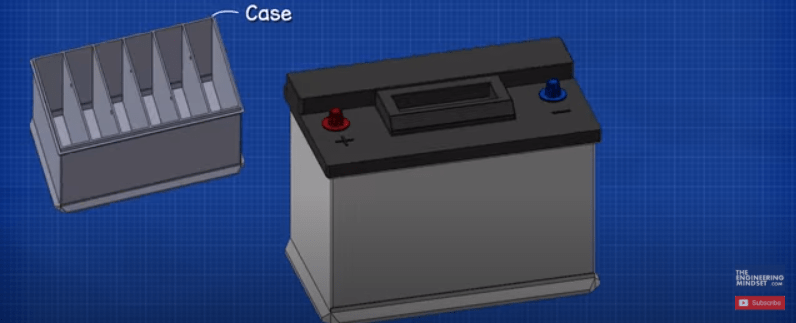
First of all, we have the plastic case which holds all the internal components in place. On the top we have the plastic lid and there are two terminals, a positive and a negative which are called the terminal posts.
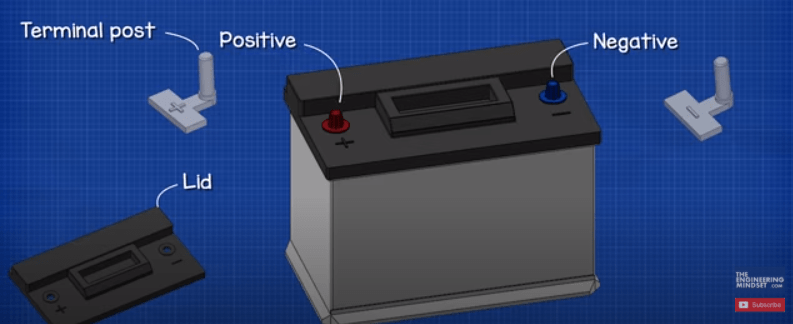
By removing the lid we can see inside. Notice the casing is divided up into 6 separate chambers, each separated by a plastic wall. Each chamber is known as a cell. Each cell generates around 2.1 volts of DC or direct current. Each cell is connected in series, the negative of one cell is connected to the positive of the next cell, to give us a total voltage of around 12.6V.
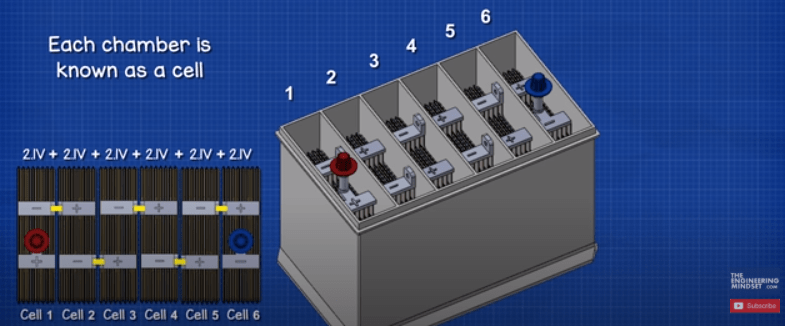
It’s the same as if you connect household alkaline batteries together, their voltages add together to provide a higher total voltage.

Each cell in the battery is connected via a plate strap, which is made from lead. These are welded together through the plastic wall to form the connection.

As we look at the battery from this view, we see that current flows through the battery cells from the positive to the negative, and that’s using conventional current theory. What actually happens is the electrons flow in the opposite direction from the negative to the positive. But we’ll cover that a little later in the article.
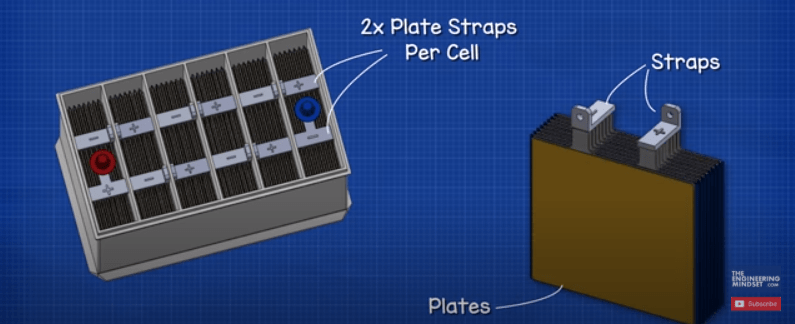
Notice there are two plate straps in each cell. One positive and one negative. These are called plate straps because each strap is connected to a number of plates, which are sheets of lead.
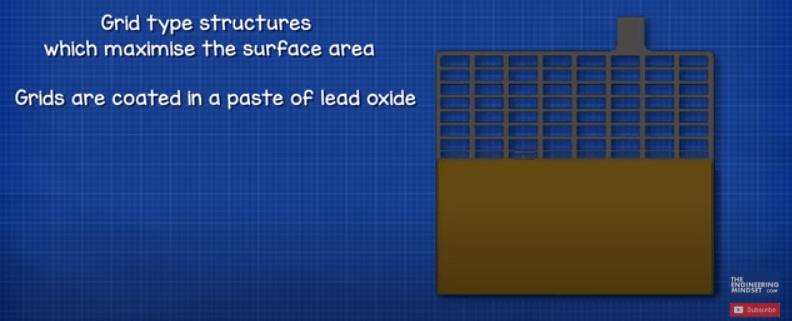
The plates are formed into grid type structures which maximise the surface area. The grids are coated in a paste of lead oxide. The paste is where the chemical reaction occurs and we’ll see that a little later in the article. The paste acts a bit like a sponge and is going to absorb some of the electrolyte liquid which improves battery performance. The size of the plate determines how much current a battery can provide, but it doesn’t change the voltage. The materials used and the number of plates determines the voltage produced by each cell. The grid holds the paste in place to ensure a uniform current distribution across the plate and helps transport the electrons out of the battery and around the electrical circuit.

The negative plate is the anode and this is a plate of pure lead, although small amounts of additives are added to harden the lead and protect it from corrosion. The positive plate is the cathode and this is made from lead oxide. The plates are made of dissimilar materials to form the chemical reaction and release electrons. We don’t want the positive and negative plates to come into contact with each other though, this would short circuit the battery. So, instead, we place each positive plate into an envelope separator. This is a porous material that allows ions to flow through without the materials coming into direct contact with each other.
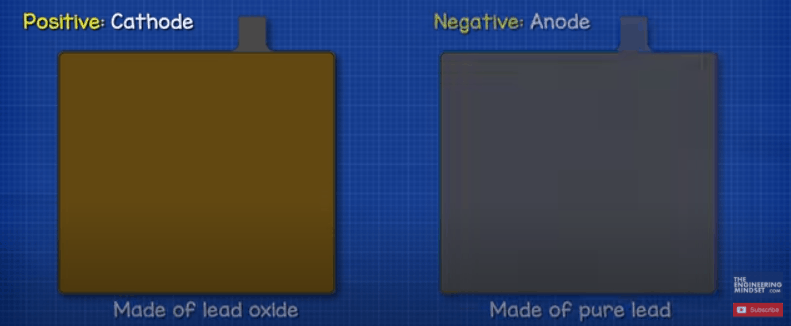
The positive and negative plates sit between each other with a small gap in-between each. The chamber is then filled with an electrolyte liquid of sulfuric acid and water. Hence the battery is called a lead acid battery.

Electricity Fundamentals
We want to quickly recap on the fundamentals of electricity so that you understand the next part of how the battery works.
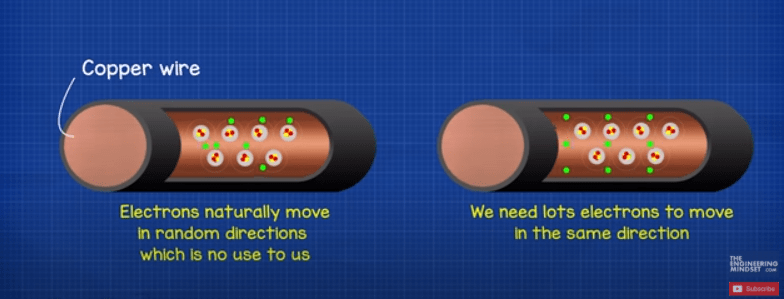
Electricity is the flow of electrons in a circuit. We need lots of electrons to flow in the same direction through a wire so we can place things in the path of the electrons such as light bulbs. The electrons will have to pass through this and as they do they produce light. When lots of electrons flow in the same direction, well call this current.
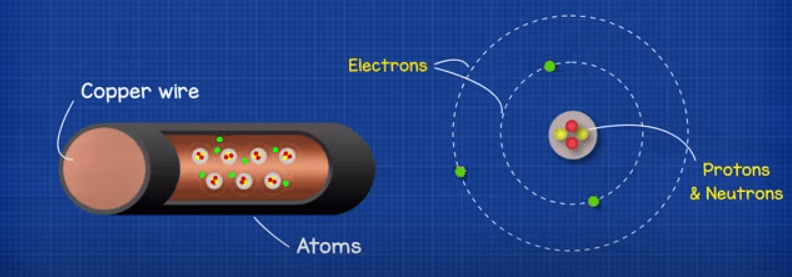
Every material is made from atoms. The atoms have different numbers of protons, neutrons and electrons which is what makes the material different. Some materials like copper have an electron which is free to move to other atoms. If we connect a power supply such as a battery to the copper wire, the voltage will push the electrons and they rush to get to the positive terminal of the battery.
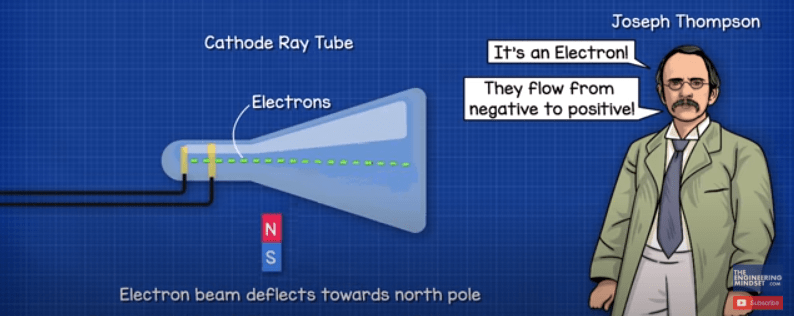
We said the electrons flow from the negative to the positive. This is known as electron flow, it’s a theory of how electricity works and it’s what’s actually occurring. But, you might be used to seeing conventional current which is from positive to negative, this is the original theory which is known as conventional current. This was proved wrong by Joseph Thompson who discovered the electron and found that they flow from the negative to the positive.
However, we still to this day use conventional current theory when designing electrical circuits. If we looked at this simple circuit we must always assume that current is flowing from positive to negative, but engineers and scientists know the electrons actually flow in the opposite direction. The electrical formulas we use will still come out with the same answers regardless of which way the electricity is flowing so it doesn’t really matter.
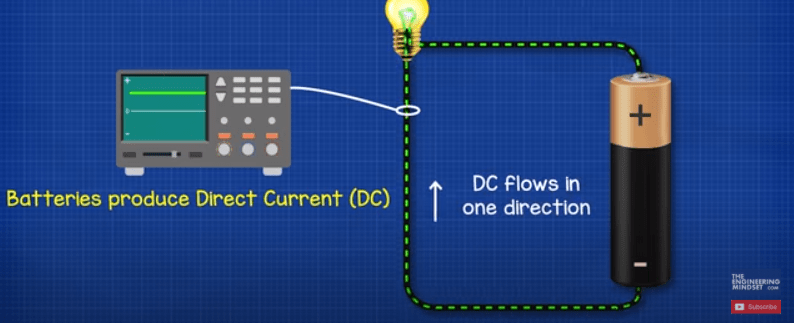
There are two types of electricity, DC direct current which we get from batteries. The electrons in this type are pushed in one direction. So it’s called direct current. Think of this like water flowing down a river. The other type of electricity is AC or alternating current which is what you get from the power outlets in your homes. In this type the electrons are pushed and pulled forwards and backwards constantly. Think of this type like the tide of the sea flowing in and out.
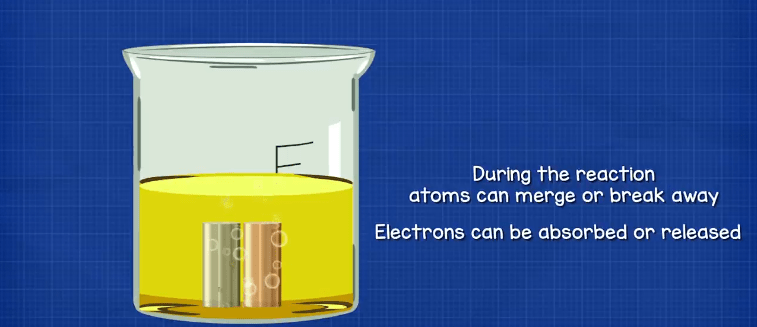
When we mix certain materials together, we can cause chemical reactions. This is when the atoms of one material interact with the atoms of another material. During this interaction atoms will bond together or break apart. Electrons can also be released or captured by atoms during the reaction.
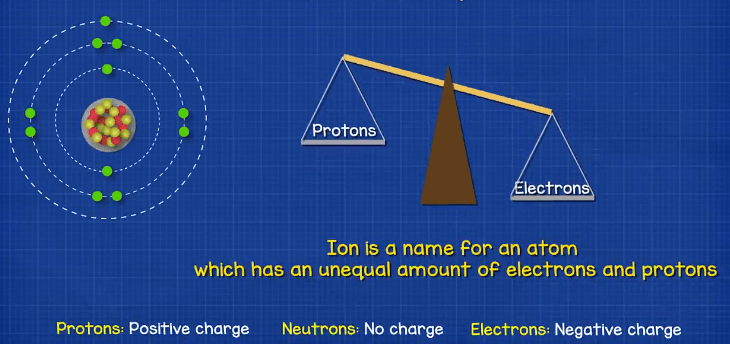
When we talk about atoms, you will usually hear the term ion used. An ion is an atom which has an unequal number of protons or electrons. An atom has a neutral change when is has the same number of protons and electrons, because the protons are positively changed and the electrons are negatively charged, so they balance out. If the atom has more electrons than protons then it’s a negative ion. If the atom has more protons than electrons it’s a positive ion.
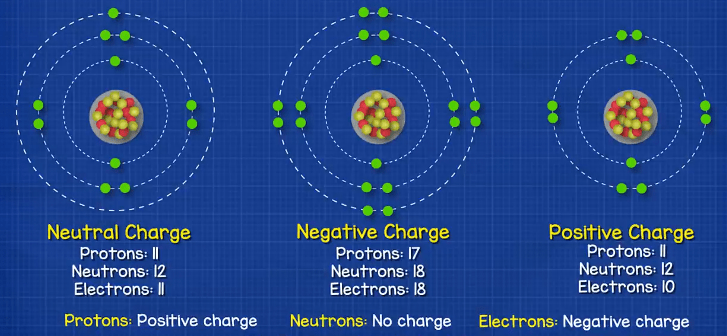
How it Works
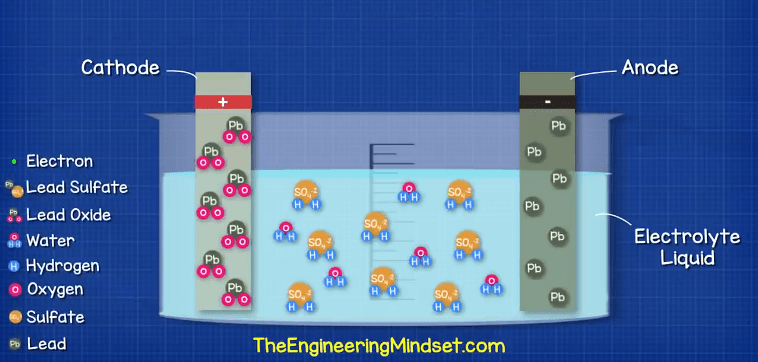
Rather than trying to understand this complex construction, we’re going to simplify it down to this simple model of a cell with a single cathode and anode.
In this cell we have the electrolyte liquid which is 1/3 sulfuric acid and 2/3 water.
We have the positive electrode which is the cathode, this is made from lead oxide (PbO2)
We then have the positive electrode which is the anode, this is made from pure lead (Pb)

When these materials are combined, we’re going to get a small chemical reaction between the atoms. We’ll show the atoms of these materials with these coloured spheres.
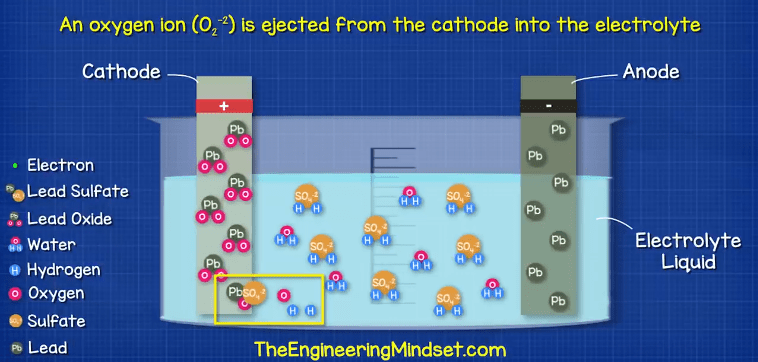
The positive cathode terminal of lead oxide (PbO2) is going to react with the sulfate (SO4-2) in the electrolyte, this will form a layer of Lead Sulfate (PbSO4) on the cathode terminal. During this reaction, an oxygen ion (O2-2) is ejected from the cathode into the electrolyte. Once in the electrolyte these oxygen ions will combine with the hydrogen ions (H+) to form water (H2O).

At the same time, the Lead atoms on the anode are going to react with the Sulfate ions (SO4-2) in the electrolyte. This reaction will create a layer of Lead Sulfate (PbSO4) around the electrode. During this reaction two electrons are released and collect in the negative terminal.
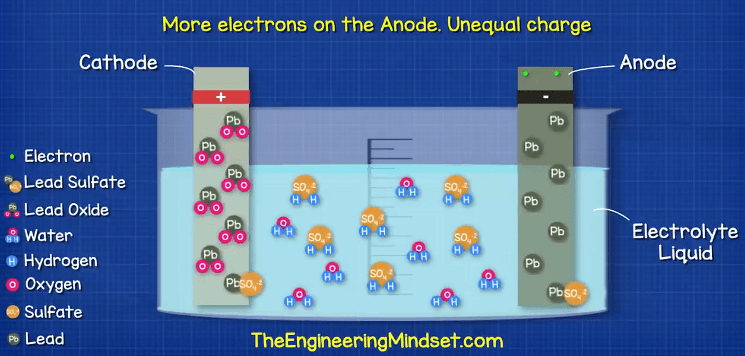
So now we have a build-up of electrons on the negative terminal. As electrons are negatively charged, this means we have a difference in charge across the two terminals and we can measure this with a voltmeter or multimeter.
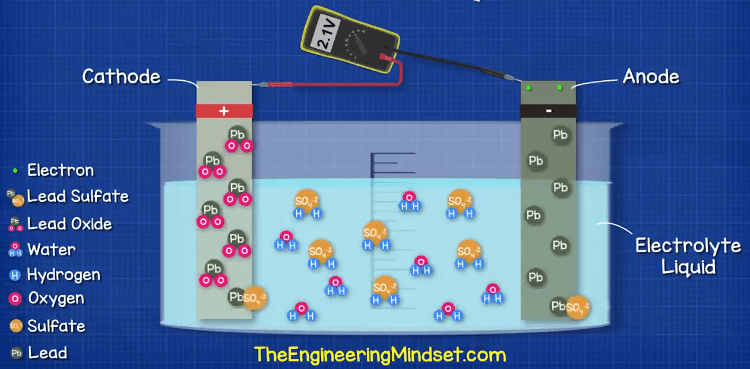
If you think about a magnet, the opposite ends attract and the alike ends repel each other. The electrons are negatively charged and so they repel each other, and they are attracted to the positive terminal which has less electrons. But, they can’t reach this. If we provide a path for the electrons, such as a wire, then the electrons will flow through this to get to the positive terminal. We can then place things such as a lamp in the way of these electrons, and use them to do work such as illuminating the lamp.
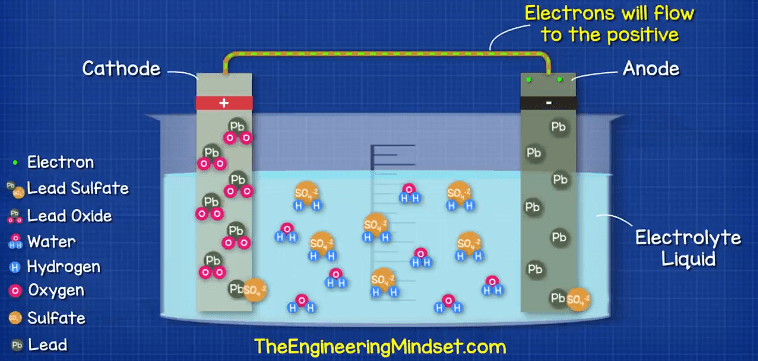
While the path exists, the chemical reaction continues, but this won’t last forever. The chemicals required for the reaction will run out. The acid becomes diluted and weaker and a build up of lead sulfate coats both electrodes, this means the materials are becoming more similar and so the chemical reaction becomes harder to achieve.
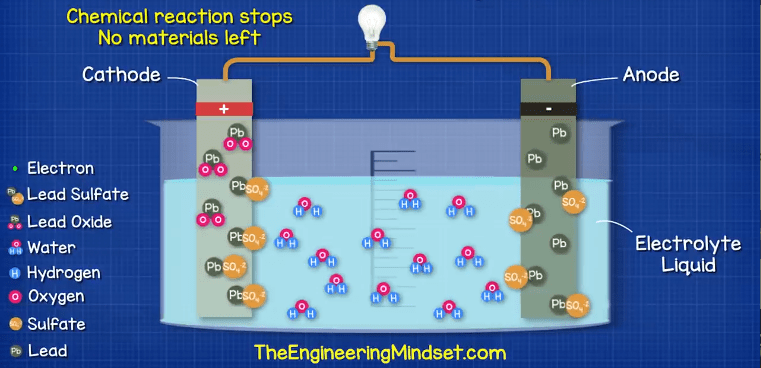
But luckily this chemical reaction can be reversed, so if we supply the battery with electricity from the alternator, we can start to reverse the reaction.

The electrons enter the negative terminal and re-join with the lead sulfate, releasing the sulfate into the electrolyte to leave just lead on the negative plate. The suflate ions enters the electrolyte and combine with the hydrogen ion to release the oxygen ion so the electrolyte acid becomes stronger. The oxygen ion combines with the lead to create lead oxide and this releases the suflate back into the electrolyte, again making it stronger.
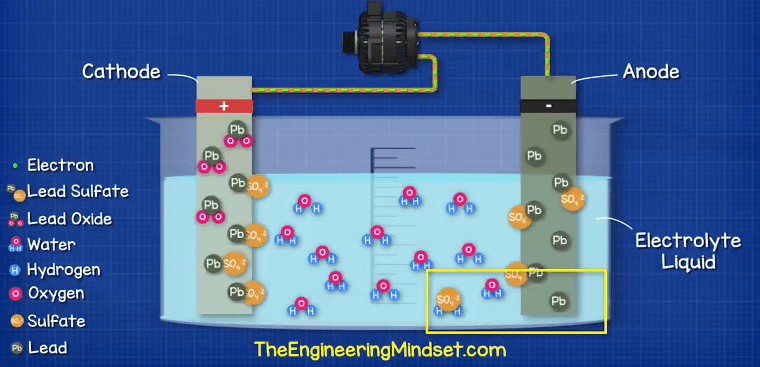
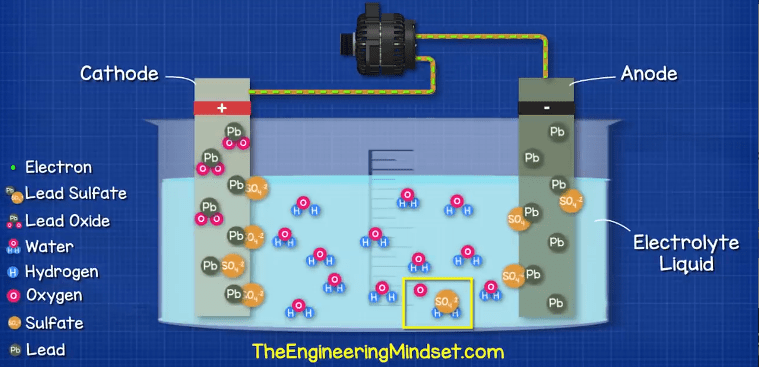
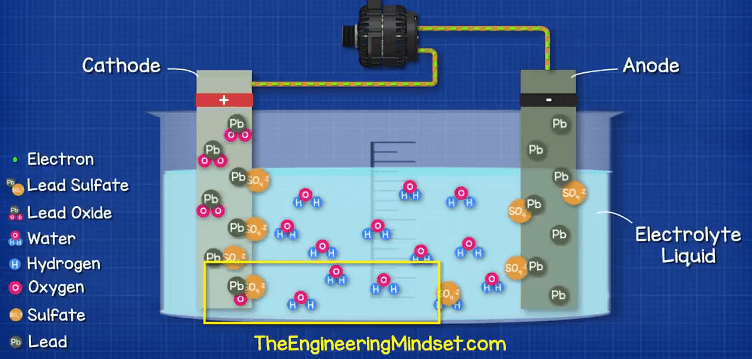
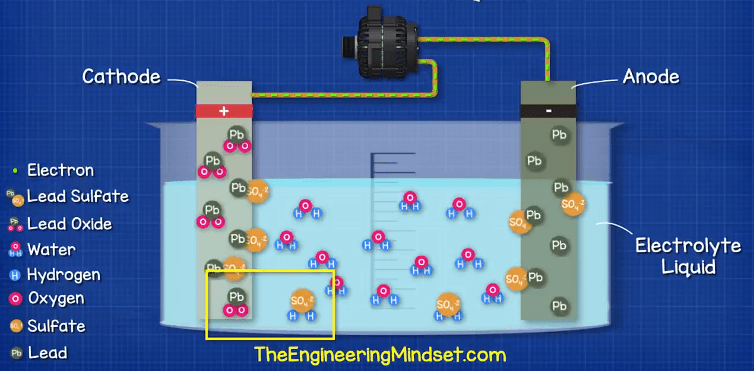
If we were to leave the battery to fully discharge for too long or too many times, it becomes very difficult to reverse the chemical reaction. Additionally, the sulfate layer could break away from the electrodes and acclimate at the bottom of the battery which will mean it no longer participates in the chemical reaction so the battery needs to be repaired or replaced.
So when we look at the battery, this chemical reaction is occurring between every plate in every cell to provide the hundreds of amps of current to start the starter motor and also provide the voltage to power the lights etc. This is then recharged by the alternator.
Testing a Car Battery With a Multimeter
To test the voltage of a car battery we simply switch to the DC voltage setting on our multimeter and then connect the red lead to the positive and the black lead to the negative. We should see a voltage of around 12.6V if it’s below 12 then the battery is not functioning properly.
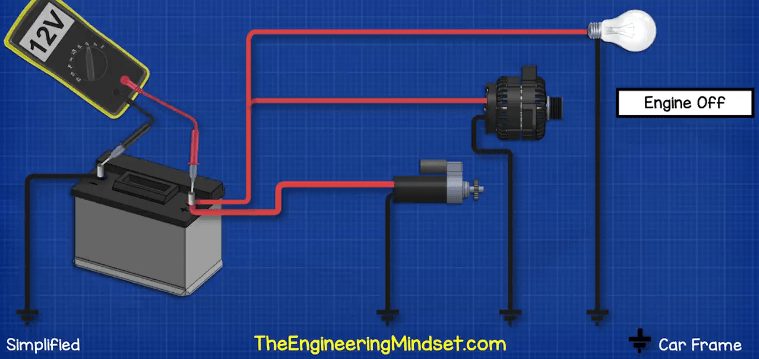
When we start the car, the voltage will drop because the starter motor is pulling a huge amount of current. The voltage will drop down to around 11 volts, if it drops below about 10 volts then the battery is not functioning properly.
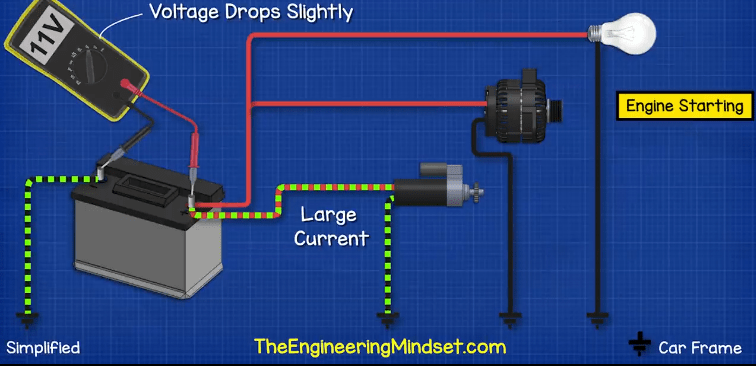
Once the engine is running, the alternator should be generating electricity and so we should see a higher voltage of around 14 volts, because the alternator is recharging the battery and the voltage needs to be higher to help force the electrons back in and reverse the chemical reaction.

But now that you’re all charged up, checkout squarespace.com to create your own online web presence which is packed with features to empower individuals to launch, share and promote their own projects.
There’s powerful blogging tools to showcase your projects photos, videos and progress updates.
You can easily schedule appointments for classes and sessions with team members and clients through their built in tool. And you can even collect payments or donations to help support your cause.
Head to squarespace.com for a free trial, and when you’re ready to launch, go to squarespace.com/engineeringmindset to save 10% off your first purchase of a website or a domain.













Hii Paul,
This is really a detailed one and it works great ..!!
can you let me know why no one wants to say what is the compound they use to hold paste to the grid on cell plates I have not been able to find it anywhere you can watch many video’s and not one person says do you know what it is I am trying to test something but if I cant keep red lead on the plate I will never find out can you help I hope it is nothing simple like Borax as it will hold with heat Cheers.
sir, i think i found a small mistake,
* how it works:-
Then we have the positive electrode which is the anode, it is made of pure lead (pb)*
I have a little doubt positive is anode or cathode
[…] We have covered how alkaline batteries work in our previous article, check that out HERE. […]
[…] in each battery there are about six cells on average. In each cell, there are lead plates that are submerged in sulfuric acid. The average lead-acid battery is able to store 12V of power. About 2V per cell. Enough to allow […]
[…] in each battery there are about six cells on average. In each cell, there are lead plates that are submerged in sulfuric acid. The average lead-acid battery is able to store 12V of power. About 2V per cell. Enough to allow […]